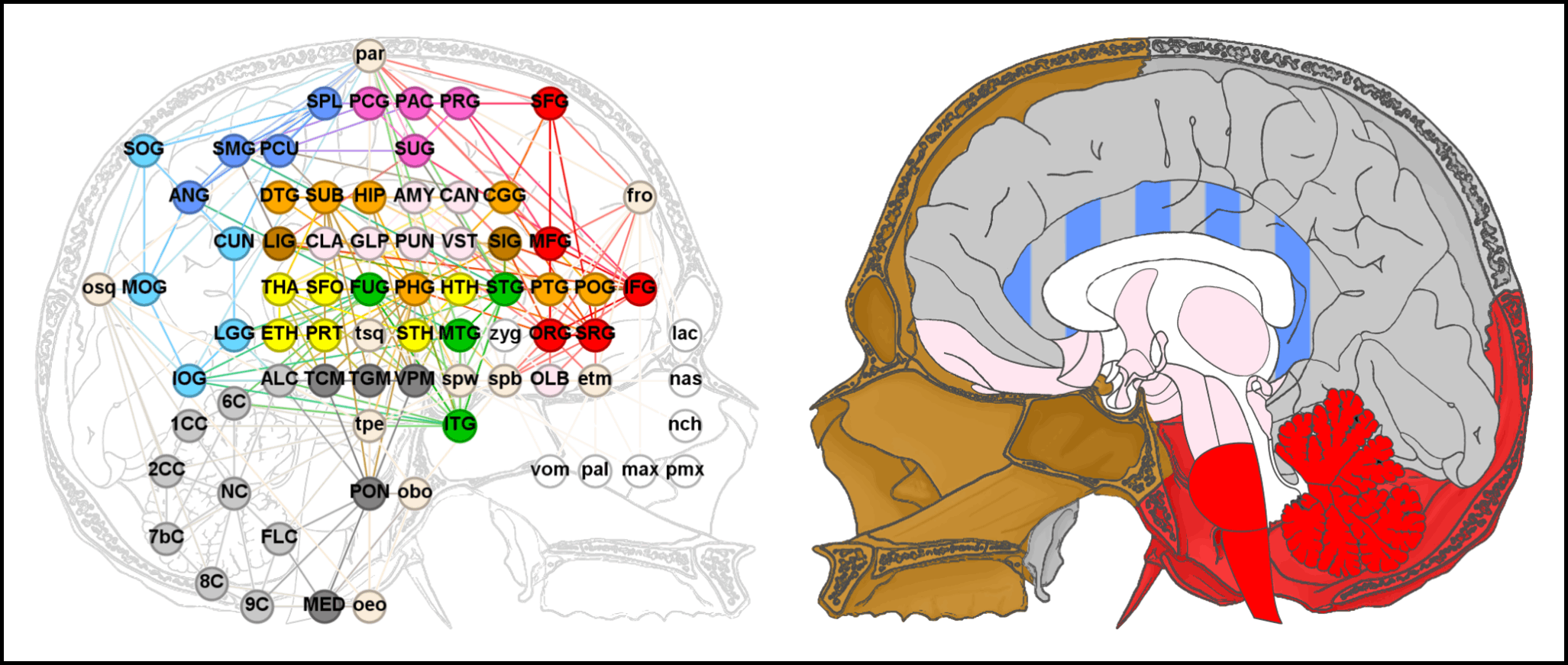
In the past year we have published a series of three studies that introduced anatomical network analysis to the brain, specifically in humans (Homo sapiens). Our aim was to highlight pivotal elements and general phenotypic patterns in the morphological organization of the human brain by investigating its intrinsic and extrinsic spatial constraints through topology—that is, patterns of physical interaction. We started with the human brain itself and found that the parahippocampal gyrus of the limbic lobe (i.e., the deep medial foil to the temporal lobe) is of great structural relevance in cerebral architecture. Then we proceeded to investigate community detection as a proxy for modularity in human brain morphology and detected the simultaneous occurrence of two modular patterns which mirror the organization of the surrounding braincase: a vertical division in line with the different ontogenetic processes associated with cranial base and vault, respectively, and a longitudinal gradient consistent with the distinct morphogenetic environments of the three cranial fossae. Lastly, to actually test whether our findings could be contextualized in light of the braincase, as we suspected, we included the skull into the model. Besides matching our expectations in regard to the anatomical system’s modularity, this study suggested that the sphenoid bone and parahippocampal gyrus are the elements of the highest structural relevance, especially due to their role as an interface between soft and hard tissues of the head.
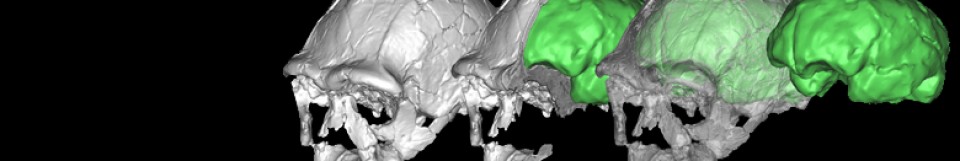
Now we have extended our analysis to a new species, the chimpanzee (Pan troglodytes), and have compared its results to our prior research (Schuurman & Bruner, 2024). Chimpanzees and humans share morphologically complex inferior-medial cerebral regions and a topological organization that corresponds to the spatial arrangement of the braincase. These mutual topological characteristics are interesting because they can probably be traced back 7-10 million years ago, to the Pan–Homo Last Common Ancestor. However, some crucial differences are found between the chimpanzee and human brains as well, namely, in the structural relevance and roles of their respective cerebral components. Most notably, in chimpanzees, the cerebellum is embedded in a more intricate topological context than the parahippocampal gyrus, even compared with humans. The structural relevance of the cerebellum is likely to stem from the lack of expansion and reorganization in the chimpanzee temporal lobes. This could have caused the forebrain to envelop the midbrain and hindbrain more notably than in humans, leading to more spatial interactions between the cerebellum and the other brain regions. We hope this information will help to interpret macroanatomical changes in the brains of fossil hominids.
Tim Schuurman