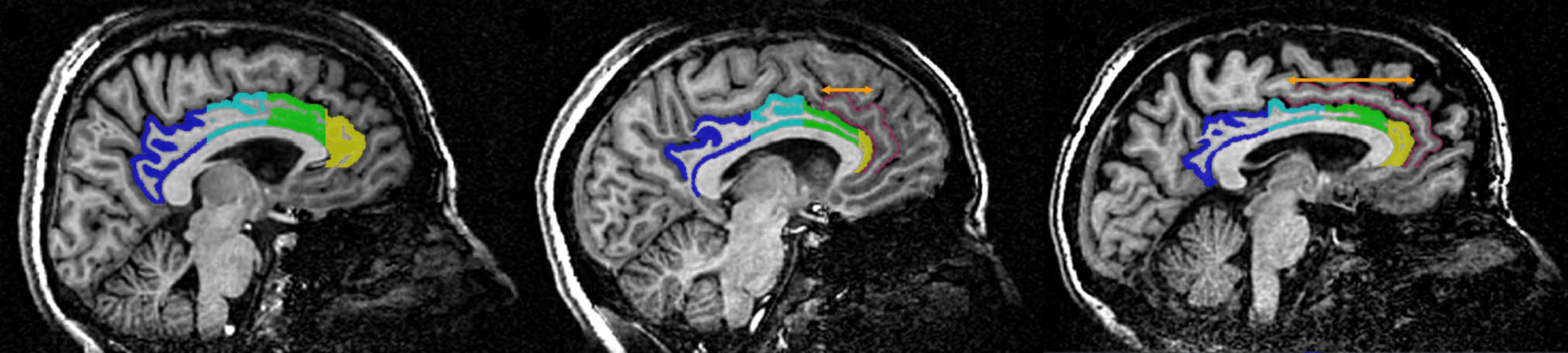
Studies using functional neuroimaging have shown that the superior parietal lobe (SPL) is involved in cognitive processes such as visuospatial integration and attention. The SPL is sometimes separated into two parts, the anterior and posterior regions, by the superior parietal sulcus (SPS). Drudik et al. (2022) conducted a study examining the morphological variability of the SPS in 40 human brain MRIs. Through volumetric and spatial probability maps, the analysis aimed at quantifying the SPS’s spatial position, following the Montreal Neurological Institute (MNI) standard stereotaxic space
When dealing with brain morphological differences, a major challenge is to provide a consistent definition of the elements involved, able to cope with the fuzzy variability of idiosyncratic anatomical features. In this case, the SPS definition, used as identification criteria, was: “the dorsal parietal sulcus located within the SPL, posterior to the superior postcentral sulcus (SPCS), and anterior to the paroccipital segment of the intraparietal sulcus (IPS-PO).” The presence of more than one branch has been interpreted as an “SPS complex.” Three categories were established: Type I for a single sulcus, Type II for multiple sulci, and Type III for a group of dimples forming a complex. Additionally, the first two SPS types were subdivided into five subcategories (a to e), based on their interaction with surrounding sulci.
Their results indicate that Type I SPS was present in 75% of the hemispheres studied, with Type II accounting for 22.5%, and Type III making up the remaining 2.5%. These results differ somewhat from Ono et al. (1990), where SPS could not even be detected in 20% of the left hemispheres and 40% of the right ones.
The deep developmentof the SPS in the precuneus would probably merit further attention. According to the study of Pereira-Pedro & Bruner (2016), in fact, this region displays irregular and variable sulcal patterns.
Rafael Gallareto